Aviation Weather
full text of the classic FAA guide
CHANGE OF STATE
Evaporation, condensation, sublimation, freezing, and melting are changes of state. Evaporation is the changing of liquid water to invisible water vapor. Condensation is the reverse process. Sublimation is the changing of ice directly to water vapor, or water vapor to ice, bypassing the liquid state in each process. Snow or ice crystals result from the sublimation of water vapor directly to the solid state. We are all familiar with freezing and melting processes.
LATENT HEAT
Any change of state involves a heat transaction with no change in temperature. Figure 35 diagrams the heat exchanges between the different states. Evaporation requires heat energy that comes from the nearest available heat source. This heat energy is known as the “latent heat of vaporization,” and its removal cools the source it comes from. An example is the cooling of your body by evaporation of perspiration.
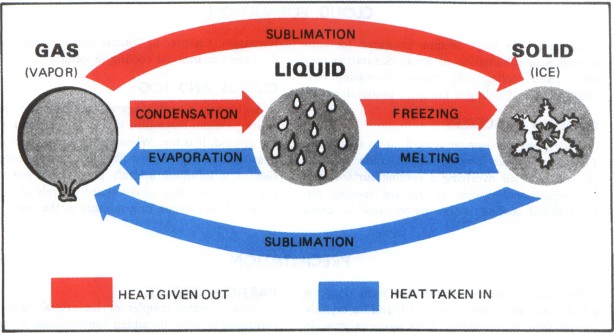
What becomes of this heat energy used by evaporation? Energy cannot be created or destroyed, so it is hidden or stored in the invisible water vapor. When the water vapor condenses to liquid water or sublimates directly to ice, energy originally used in the evaporation reappears as heat and is released to the atmosphere. This energy is “latent heat” and is quite significant as we learn in later chapters. Melting and freezing involve the exchange of “latent heat of fusion” in a similar manner. The latent heat of fusion is much less than that of condensation and evaporation; however, each in its own way plays an important role in aviation weather.
As air becomes saturated, water vapor begins to condense on the nearest available surface. What surfaces are in the atmosphere on which water vapor may condense?
CONDENSATION NUCLEI
The atmosphere is never completely clean; an abundance of microscopic solid particles suspended in the air are condensation surfaces. These particles, such as salt, dust, and combustion byproducts are “condensation nuclei.” Some condensation nuclei have an affinity for water and can induce condensation or sublimation even when air is almost but not completely saturated.
As water vapor condenses or sublimates on condensation nuclei, liquid or ice particles begin to grow. Whether the particles are liquid or ice does not depend entirely on temperature. Liquid water may be present at temperatures well below freezing.
SUPERCOOLED WATER
Freezing is complex and liquid water droplets often condense or persist at temperatures colder than 0° C. Water droplets colder than 0° C are supercooled. When they strike an exposed object, the impact induces freezing. Impact freezing of supercooled water can result in aircraft icing.
Supercooled water drops very often are in abundance in clouds at temperatures between 0° C and −15° C with decreasing amounts at colder temperatures. Usually, at temperatures colder than −15° C, sublimation is prevalent; and clouds and fog may be mostly ice crystals with a lesser amount of supercooled water. However, strong vertical currents may carry supercooled water to great heights where temperatures are much colder than −15° C. Supercooled water has been observed at temperatures colder than −40° C.
DEW AND FROST
During clear nights with little or no wind, vegetation often cools by radiation to a temperature at or below the dew point of the adjacent air. Moisture then collects on the leaves just as it does on a pitcher of ice water in a warm room. Heavy dew often collects on grass and plants when none collects on pavements or large solid objects. These more massive objects absorb abundant heat during the day, lose it slowly during the night, and cool below the dew point only in rather extreme cases.
Frost forms in much the same way as dew. The difference is that the dew point of surrounding air must be colder than freezing. Water vapor then sublimates directly as ice crystals or frost rather than condensing as dew. Sometimes dew forms and later freezes; however, frozen dew is easily distinguished from frost. Frozen dew is hard and transparent while frost is white and opaque.
To now, we have said little about clouds. What brings about the condensation or sublimation that results in cloud formation?
Table of Contents
Previous Section: Water Vapor
Next Section: Cloud Formation
A PDF version of this book is available here. You may be able to buy a printed copy of the book from amazon.com.
